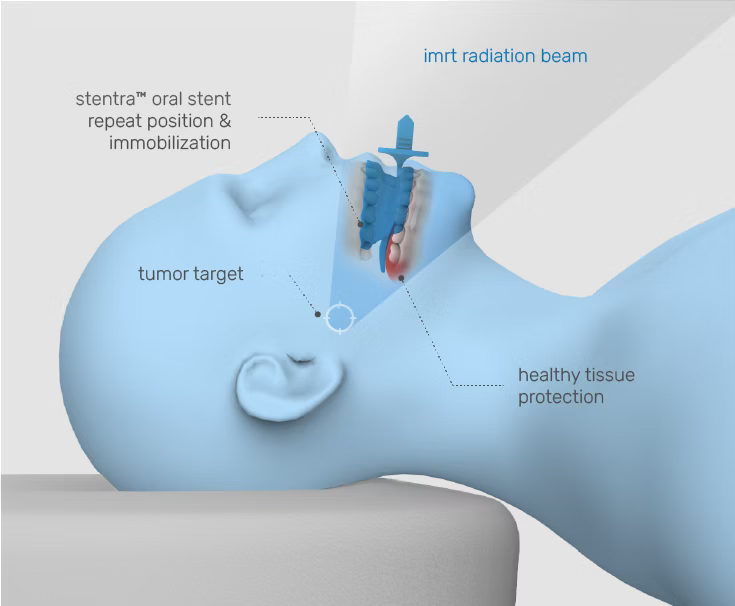
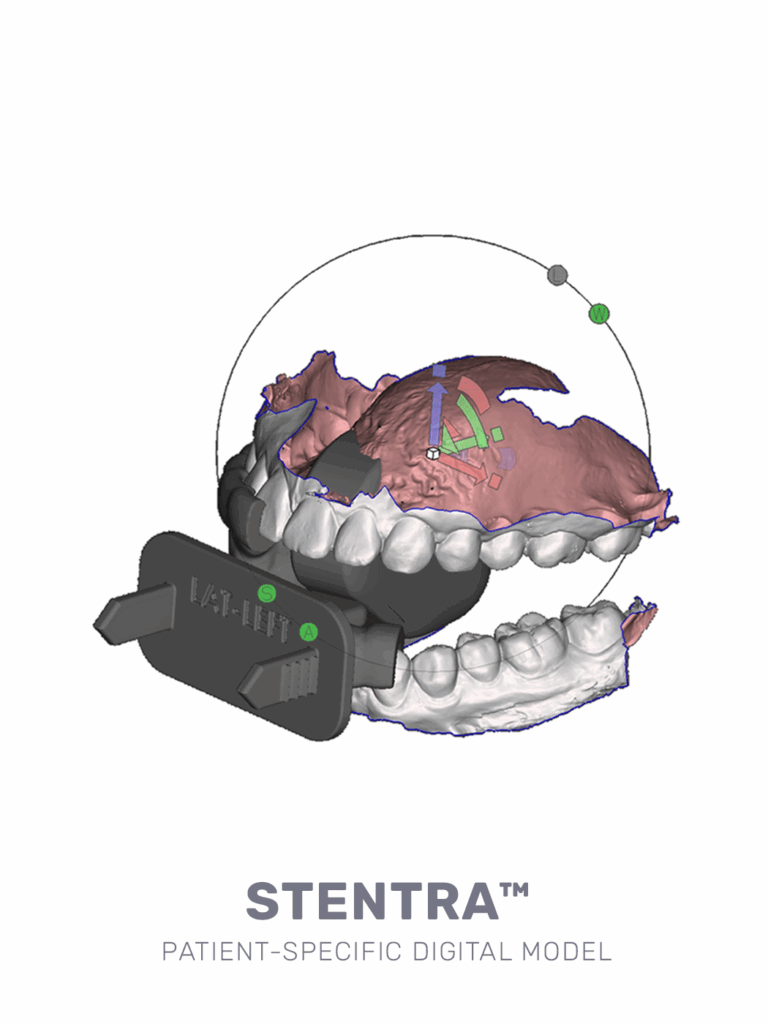
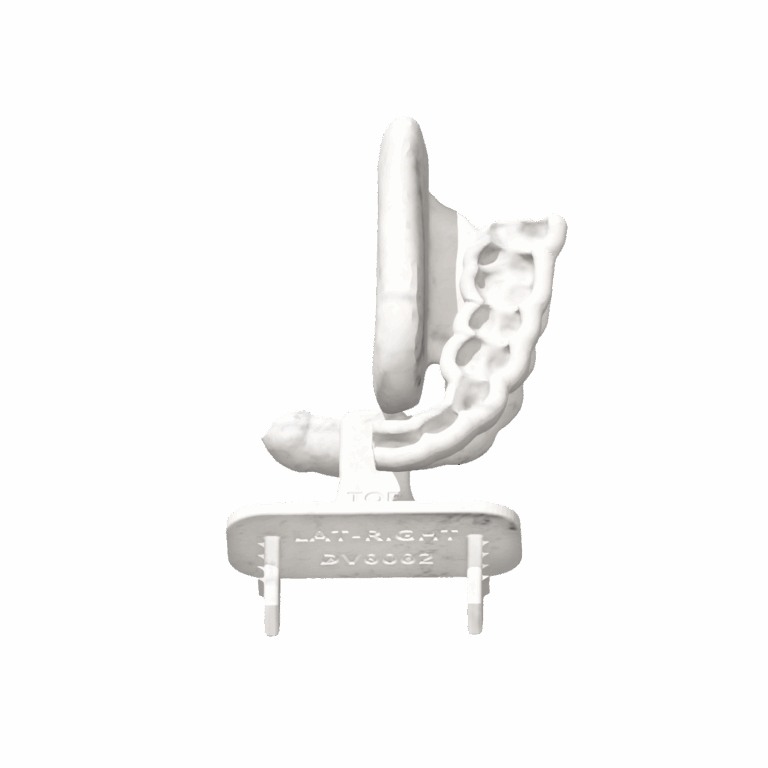
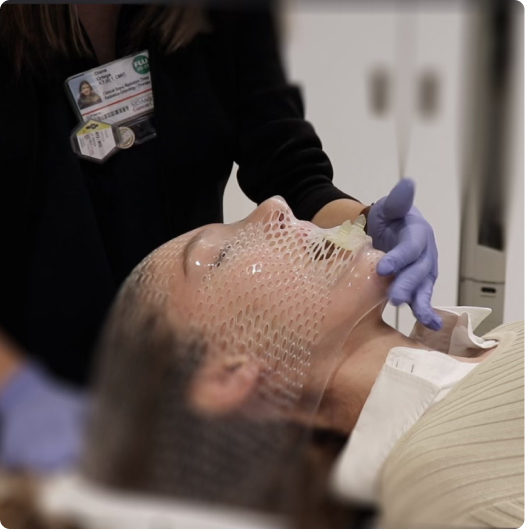
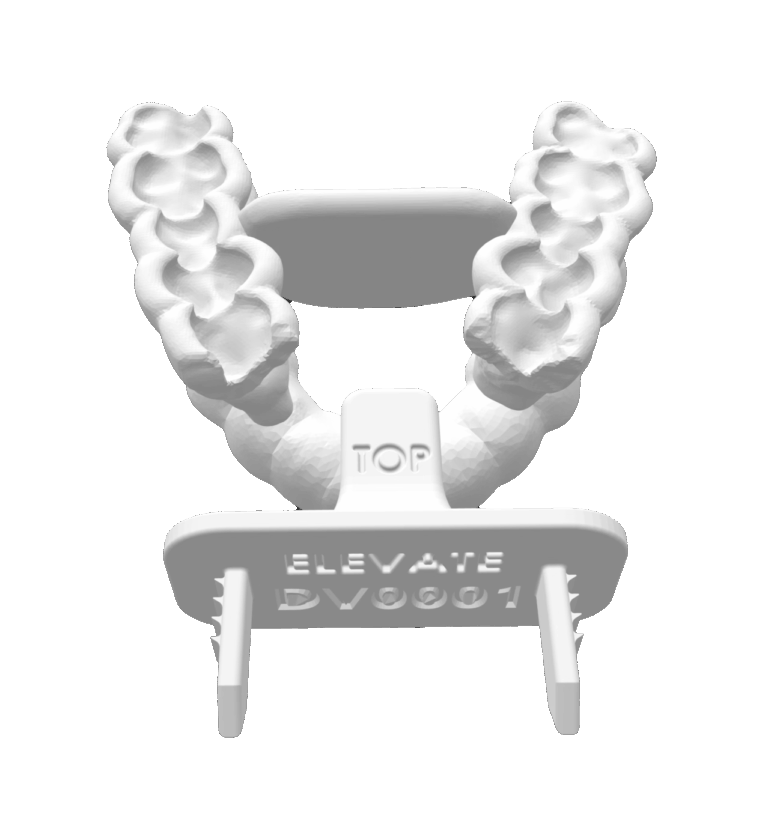personalized protection— precision where it counts
Stentra™ delivers custom devices designed to fit your patient’s anatomy – helping with radiation targeting accuracy while protecting healthy tissues and reducing side effects.
- CPT-reimbursable
- sub-millimeter precision
radiation should treat cancer— not compromise healthy tissue
Inconsistent immobilization leads to clinical inefficiencies and patient risk
When targeting is imprecise, the likelihood of side effects such as mucositis increases — leading to treatment interruptions, re-planning burdens, and diminished therapeutic outcomes.
precision-built for your patient, streamlined for your workflow
- custom-crafted from intraoral scan
- ready for use in 72 hours
- reimbursable & HIPAA compliant
- configurable tongue positioning for optimal setup
- reusable with validated cleaning protocol
our community




why precision matters
One size does not fit all. Behind every personalized solution lies a proven reduction in patient pain, treatment delays, and clinical workflow complexity — all supported by real-world data.
reduce mucositis
severity1
decrease radiation to
tongue area2
diminish difficulty
swallowing3
1 Are intraoral stents effective for reducing the severity of oral mucositis during radiotherapy for maxillary and nasal cavity cancer? (2020), Journal of Oral and Maxillofacial Surgery
2 Tongue displacement device in decreasing the radiation dose to tongue and preventing proton beam overshoot (2021), Frontiers in Physics
3 The impact of tongue-deviating and tongue-depressing oral stents on long-term radiation-associated symptoms (2020), Clinical and Translational Radiation Oncology
from scan to simulation
in 3 simple steps
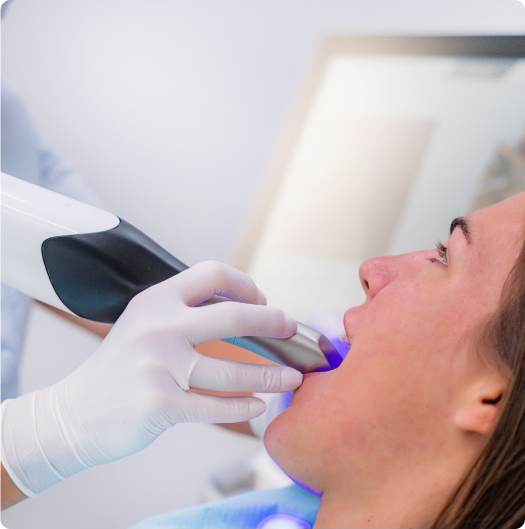
Capture a precise 3D model of your patient’s oral anatomy in under 15 minutes using a standard intraoral scanner. Scans are uploaded to our secure portal, enabling same-day design initiation and streamlined workflow


no more one-size-fits-all
no more avoidable suffering
leading centers using stentra
We collaborate with top-tier medical centers across the U.S. and Europe to bring personalized oral protection into real-world oncology workflows. Together, we turn innovation into standard care — one patient at a time.
frequently asked
questions
Is Stentra™ reimbursable under existing CPT codes?
Yes. Stentra™ is eligible for reimbursement using current CPT codes for radiation therapy immobilization devices—typically CPT 77334 for custom, complex devices. Reimbursement amounts will vary based on payer policies, correct code selection, and supporting documentation. We supply clients with onboarding tools including coding guidance and claim-ready documentation templates to optimize codability and reimbursement.
What 3D printing process and materials are used to manufacture Stentra™?
Stentra™ devices are manufactured using a high-resolution additive manufacturing process (3D printing) with a biocompatible, medical-grade resin. This approach ensures clinical precision, consistent reproducibility, and a smooth surface finish—critical for intraoral comfort, safety, and anatomical conformity.
Does the presence of the immobilization & positioning device impact the radiation beam or planning accuracy?
No. Stentra™ is fabricated from low-density, non-metallic materials that are radiographically visible on CT but do not attenuate or scatter the therapeutic radiation beam. It integrates seamlessly into standard treatment planning workflows and does not require dose modification or beam compensation, preserving planning accuracy and clinical efficiency.
How should the Stentra™ device be reprocessed between treatment sessions?
Stentra™ is designed for repeated use throughout the patient’s treatment course. Each device is provided with a validated Instructions for Use for reprocessing and a dedicated reusable storage container. Radiation therapists can safely clean, disinfect, and store the device between sessions by following the recommended procedures—maintaining device integrity, safety, and patient comfort.
Will radiation affect more than just the cancer?
Yes. Although radiation therapy is designed to target cancerous tissue, it can also impact nearby healthy structures — particularly in the head and neck region. Common side effects include oral pain, mouth sores, difficulty swallowing, dry mouth, and changes in speech. These effects can interfere with daily life, make it harder to complete treatment, and may persist even after therapy ends.
How does Kallisio’s mission to deliver precision therapeutic devices translate into better patient care today?
Kallisio’s mission to deliver precision therapeutic devices directly translates into better patient care by enabling clinicians to provide highly targeted, anatomically accurate, and patient-specific support during treatment. Our digital platform combines clinical insight, design automation, and rapid 3D manufacturing to produce personalized devices—like Stentra™—that improve treatment precision, and enhance patient comfort and adherence. This integration of technology and care helps minimize delays, optimize clinical outcomes, and support a more compassionate, effective treatment experience from day one.
What makes Kallisio’s intraoral platform different from other immobilization or tissue-protection tools on the market?
Kallisio’s Stentra™ platform stands apart by offering true personalization through millimeter-accurate, 3D-printed devices designed from each patient’s unique anatomy and treatment plan. Unlike prefabricated or semi-custom tools, Stentra™ not only immobilizes but also displaces critical structures such as the tongue, optimizes oral aperture, and enhances radiation delivery precision. Our simulation-ready devices are delivered within 72 hours, require no additional software, integrate seamlessly into existing workflows, and are CPT-reimbursable—enabling scalable, high-precision care without disrupting clinical operations.
What clinical expertise informs the development of Kallisio’s technology?
Kallisio’s platform was co-developed with clinical experts at leading institutions, including MD Anderson Cancer Center, and is informed by deep expertise in radiation oncology, maxillofacial prosthodontics, and medical physics. The technology behind our flagship product, Stentra™, is FDA-cleared and built on rigorously validated clinical protocols. Our multidisciplinary team brings decades of experience in oncology, digital health, and advanced manufacturing to ensure each solution meets the highest standards of patient care and regulatory compliance.
Is the Kallisio Clinical Portal HIPAA-compliant?
Yes. The Kallisio Clinical Portal is fully HIPAA-compliant. It is designed to neither collect, store, nor transmit Protected Health Information. Each case is managed using a system-generated unique device identifier, ensuring patient confidentiality while maintaining institutional control and traceability.
Why choose Stentra™
Stentra™ is the only FDA-cleared, fully patient-specific intraoral immobilization solution designed for head and neck radiotherapy. Engineered for precision, it offers rapid production and delivery within 72 hours and customizable configurations to accommodate individual tumor anatomy and treatment plans. Clinically validated and available for immediate use.