therapy is tough, we're here to help you
At Kallisio, we support head and neck cancer patients by providing custom-fit oral devices designed to make radiation therapy more comfortable and precise. Each device is tailored to your unique anatomy to help reduce side effects and support better treatment outcomes, so you can focus on healing.
radiation side effects shouldn't derail your therapy
Radiation treatment for head and neck cancer can cause painful mouth sores, difficulty eating, and dry mouth, all due to unintended damage to healthy tissue. But it doesn’t have to be this way. With the right support, these side effects can be minimized to help you stay on track with your treatment.
a personalized solution to help
you finish treatment strong
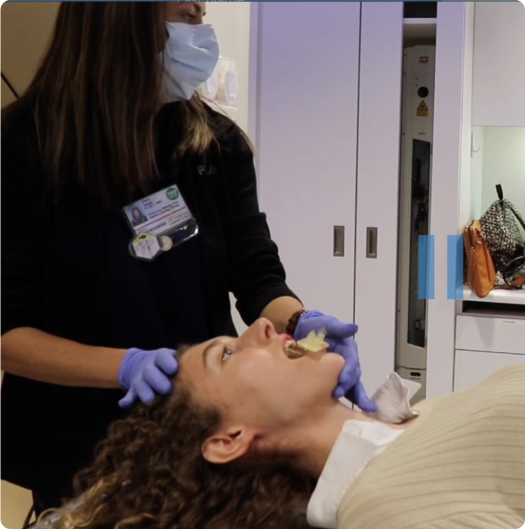
- Custom-made to fit your mouth for a secure and comfortable experience
- Moves healthy tissues out of the way during each treatment session
- Helps target the therapy dose more precisely to where it’s needed most
- Clinically shown to reduce side effects, including mouth sores and dry mouth
our community




reduction in severe oral mucositis thanks to patient-specific intraoral devices
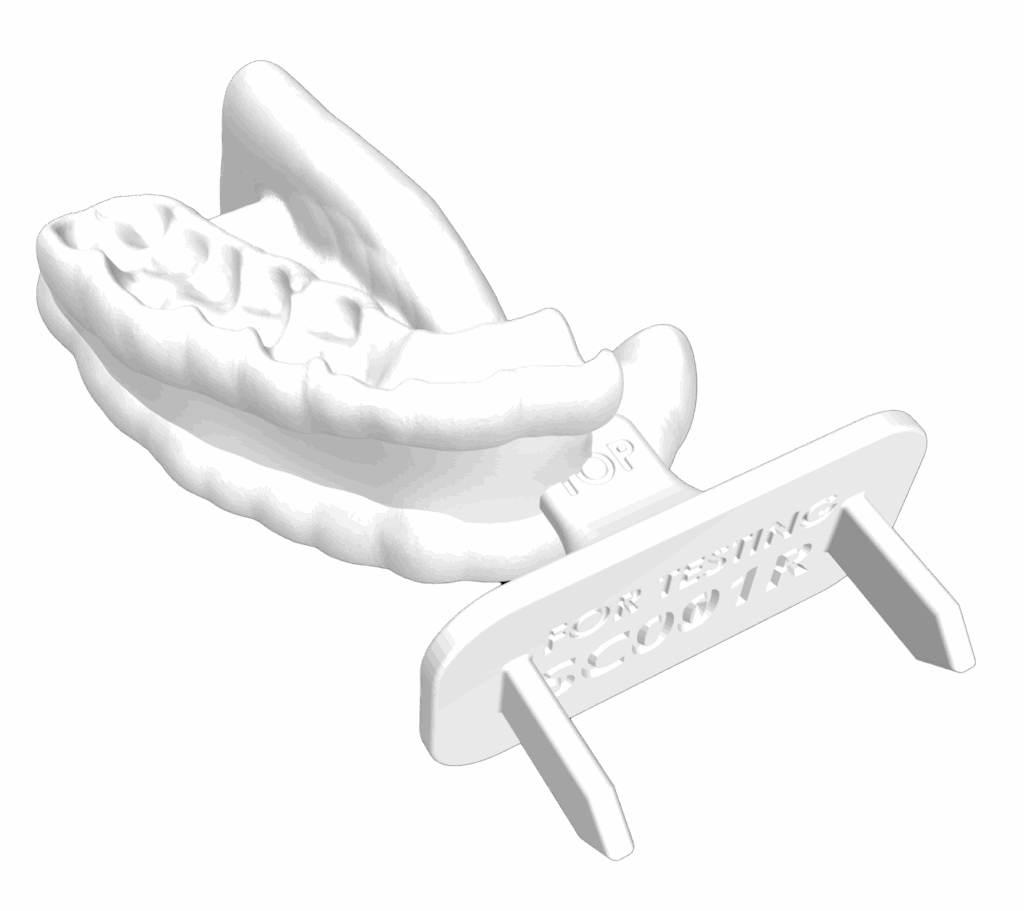
receive highly targeted therapy with
maximum precision & comfort

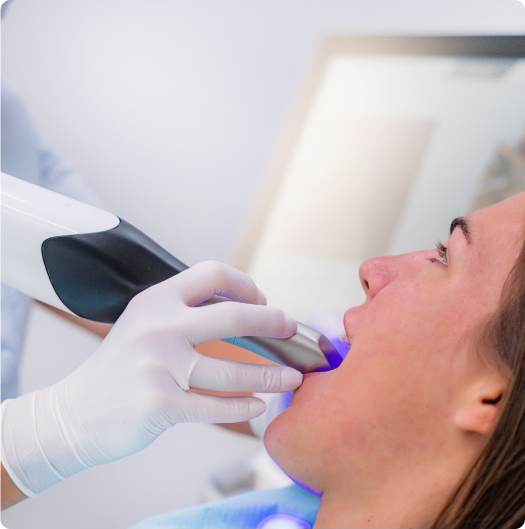

real stories,
real relief
Andrew Davis
Oncologist at MD Anderson Cancer Center
“With Stentra, I have greater peace of mind knowing we’re minimizing toxicity and reducing treatment interruptions. It helps us stay on track without compromising patient comfort. With Stentra, I have greater peace of mind knowing we’re minimizing toxicity and reducing treatment interruptions. It helps us stay on track without compromising patient comfort.


Paola Thomson
Throat Cancer Survivor
“With Stentra, I have greater peace of mind knowing we’re minimizing toxicity and reducing treatment interruptions. It helps us stay on track without compromising patient comfort. With Stentra, I have greater peace of mind knowing we’re minimizing toxicity and reducing treatment interruptions. It helps us stay on track without compromising patient comfort.
real stories,
real relief
“The staff is surprised by how many oral issues I don’t have! I’m deeply grateful to everyone for collaborating and truly hope many more patients will benefit from this new device in the future.


Paola Thomson
Throat Cancer Survivor
“With Stentra, I have greater peace of mind knowing we’re minimizing toxicity and reducing treatment interruptions. It helps us stay on track without compromising patient comfort. With Stentra, I have greater peace of mind knowing we’re minimizing toxicity and reducing treatment interruptions. It helps us stay on track without compromising patient comfort.
leading centers using stentra
We collaborate with top-tier medical centers across the U.S. and Europe to bring personalized oral protection into real-world oncology workflows. Together, we turn innovation into standard care — one patient at a time.
Kallisio follows closely the mission of patient organizations
We believe patients should be informed and supported. That’s why we work with advocacy groups to raise awareness around oral pathologies, treatment-induced side effects, and solutions that make a difference.






frequently asked questions
Is there anything I can do to protect my mouth during treatment?
Yes. Custom-fit devices like Stentra™ are designed to help protect healthy areas of your mouth during radiation therapy. Stentra™ positions your tongue and jaw to minimize exposure to radiation-sensitive tissues and keeps your mouth stable throughout each session. This can help reduce side effects such as oral pain, mucositis, and jaw stiffness. It is personalized to your anatomy and often covered by insurance.
Who is Stentra™ designed for?
Stentra™ is designed for patients undergoing radiation therapy for head and neck cancers. It is particularly beneficial for those who need precise tongue and jaw positioning during treatment, including individuals with complex anatomies or without natural teeth (edentulous). Each device is customized to the patient’s unique anatomy to help improve the accuracy of radiation delivery and reduce exposure to healthy tissues. Ask your doctor if Stentra™ may be appropriate for your treatment plan.
What makes stentra™ different from other oral immobilization & positioning device?
Unlike standard, one-size-fits-all devices, Stentra™ is fully customized to your unique anatomy using advanced 3D intraoral scanning. This personalized design ensures a more precise fit, greater comfort, and improved treatment accuracy, helping to reduce side effects by minimizing radiation exposure to healthy tissues. Ask your care team if Stentra™ is right for you.
What evidence supports stentra™ effectiveness?
Stentra™ is based on clinical research showing that customized oral stents can improve the precision of radiation delivery, reduce exposure to healthy tissues, and lessen treatment-related side effects. Studies also highlight improvements in patient comfort and treatment compliance, especially for those undergoing head and neck radiation therapy. Talk to your doctor about how Stentra™ may support your care.
Can Stentra™ be used after first radiation treatments?
Yes. While it’s ideal to begin using Stentra™ at the start of your radiation therapy, it can still be introduced after initial sessions to help protect healthy tissue and enhance comfort during the remainder of treatment. Ask your doctor to determine if Stentra™ is appropriate for your specific care plan.
How should Stentra™ be stored and reused between treatments ?
Stentra™ is designed to be reused throughout your course of radiation therapy. Each device comes with a reusable storage container and is supported by a validated cleaning and handling guide. Your radiation therapy team will clean and store the device between sessions according to these instructions—ensuring it remains safe, effective, and comfortable for continued use. Ask your care team if you have any questions about how your Stentra™ is being maintained.
How long does it take to receive a custom device?
After your oral scan is submitted, your custom Stentra™ stent is designed, 3D printed and delivered within 72 hours. It’s typically ready before your first radiation treatment session, ensuring there’s no delay in your care.
Do you ship internationally?
Yes. We work with international partners and can adapt shipping and support services to meet regional requirements. Please contact us to confirm availability and service options in your country.
When should I talk to my doctor about this?
As early as possible — ideally before your simulation scan or the start of radiation treatment. This is when your care team plans how therapy will be delivered. Bringing up options like Stentra™ early ensures your comfort, safety, and long-term quality of life are part of the conversation. Use this guide to help start that discussion.
What are the benefits of participating in the Stentra™ Proof of Value Program?
The program offers your team direct experience with Stentra™—Kallisio’s FDA-cleared, patient-specific oral stent for head and neck radiation therapy. It enables evaluation of clinical benefits such as improved patient positioning, potential reduction in oral mucositis, and enhanced treatment tolerance. Additionally, your team can assess workflow integration, billing feasibility using existing CPT codes, and potential long-term return on investment.
What’s required from my clinic to get started in the Stentra™ Proof of Value Program?
Select up to 10 head and neck cancer patients and assign a team member to upload cases via Kallisio’s secure clinical portal. An intraoral scanner is preferred—Kallisio can assist if one is not available. Device fit is verified during CT simulation. Follow-up is required to share toxicity and setup reproducibility data, which are essential to assessing clinical and operational impact.