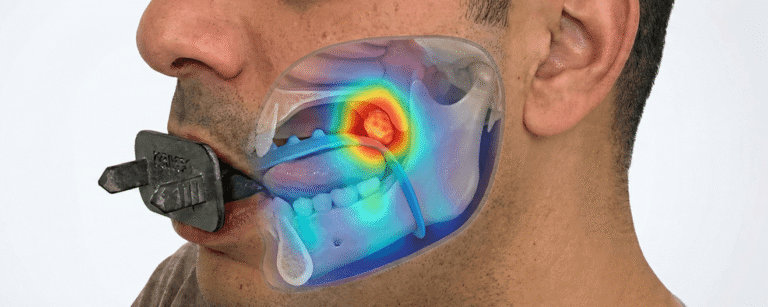
Kallisio has achieved a major milestone in its mission to transform cancer care with the recent FDA 510(k) clearance of Stentra™, its patient-specific 3D-printed intraoral immobilization and positioning device designed for radiotherapy in head and neck cancers.
The device, tailored for each patient through digital dental impressions, aims to optimize the positioning of anatomical structures during radiation therapy, minimizing exposure to healthy tissues and improving treatment precision.
According to NS Medical Devices, “Stentra offers a customizable solution designed to reduce treatment side effects such as oral mucositis, xerostomia, and trismus.”
MassDevice highlights the clinical motivation behind the innovation: “By immobilizing and displacing non-target tissues, Stentra significantly enhances radiation therapy accuracy,” reducing collateral damage to critical areas like the tongue and salivary glands.
The clearance marks a turning point in personalized oncology. As reported by Cancer Network, “The device provides physical spacing between healthy tissues and tumor targets, thereby potentially lowering radiation-induced toxicities.”
Leveraging 3D printing and digital workflows, Kallisio streamlines the traditionally manual device fabrication process. 3DPrint.com notes, “Stentra represents a convergence of precision medicine and additive manufacturing, enabling rapid production of individualized devices.”
The company’s commitment to scalability and clinical accessibility also caught the attention of 3Dnatives, which stated: “Kallisio has developed an end-to-end workflow from imaging to delivery in under a week—a crucial feature in time-sensitive cancer treatments.”
Echoing the industry excitement, 3D Printing Industry concluded: “With this FDA clearance, Kallisio is paving the way for next-generation radiation therapy, setting a precedent for customized medical devices in oncology.”
With Stentra now FDA-cleared, Kallisio positions itself as a leading innovator in personalized cancer care, with ambitions to scale its solution internationally in the coming year.